Breaking News
NRXP to File NDA for NRX-101 in Treatment of Bipolar Depression with Akathisia or Suicidality
San Mateo, CA – NRXP, a clinical-stage biopharmaceutical company, today announced plans to submit a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy Designation and Priority Review with the United States Food and Drug Administration (FDA) for NRX-101 in the treatment of bipolar depression with akathisia or suicidality.
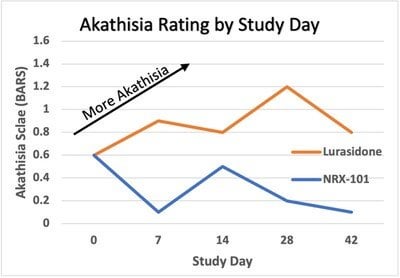
The NDA filing is based on the results of NRXP’s Phase 2b/3 and STABIL-B clinical trials, which showed statistically significant improvements in depression scores and suicidal ideation in patients with bipolar depression who were experiencing akathisia or suicidality. Akathisia, a common and debilitating side effect of antipsychotic medications, can exacerbate symptoms of depression and increase the risk of suicide in individuals with bipolar disorder.
"Today’s announcement marks a significant milestone in our journey to bring NRX-101 to patients in need," said Eric Kresh, Chief Executive Officer of NRXP. "We believe that NRX-101 has the potential to transform the treatment of bipolar depression with akathisia or suicidality by providing a new and innovative solution to improve patient outcomes."
NRX-101 is an investigational novel use of the known active ingredient oxazepam, a GABA receptor agonist. It is being developed as a potential fast-acting, once-daily treatment for bipolar depression with akathisia or suicidality. The Breakthrough Therapy Designation was granted by the FDA based on NRXP’s clinical data demonstrating NRX-101’s potential to represent a significant improvement in the treatment of patients with this serious and debilitating condition.
"The filing of our NDA for NRX-101 is a testament to our company’s commitment to advancing the treatment of serious and neglected mental health conditions," added Dr. David Peters, Chief Medical Officer of NRXP. "We are confident in the strength of our clinical data and believe that NRX-101 has the potential to become a new standard of care for patients with bipolar depression who are at risk of suicidal ideation and behaviors."
NRXP is currently completing the STABIL-B confirmatory Phase 3 clinical trial of NRX-101 in over 300 patients with bipolar depression with akathisia or suicidality. The company plans to provide further updates on the clinical program and regulatory filing process in the coming months.
About NRXP
NRXP is a clinical-stage biopharmaceutical company focused on the development of innovative treatments for serious and neglected mental health conditions. The company’s lead candidate, NRX-101, is a potential fast-acting, once-daily treatment for bipolar depression with akathisia or suicidality.
KEYWORDS
NRXP, NRX-101, Breakthrough Therapy Designation, Priority Review, New Drug Application, NDA, Bipolar Depression, Akathisia, Suicidality, Mental Health, Anxiety Disorders, Mood Disorders, Psychiatry, Neuroscience, Clinical Trials.
RELATED ARTICLES
- NRXP Presents Positive Topline Results from Phase 2b/3 and STABIL-B Trials of NRX-101 in Bipolar Depression with Akathisia or Suicidality (July 2022)
- FDA Grants Breakthrough Therapy Designation to NRXP’s NRX-101 for Treatment of Bipolar Depression with Akathisia or Suicidality (May 2022)
- NRXP Receives IND Clearance from FDA for NRX-101, a Potential Fast-Acting Treatment for Bipolar Depression with Akathisia or Suicidality (April 2021)
$NRXP – Company plans to file a New Drug Application (NDA) for Accelerated Approval under Breakthrough Therapy Designation and Priority Review of NRX-101 in treatment of bipolar depression in people akathisia or suicidality, based on the Phase 2b/3 and STABIL-B data
https://finance.yahoo.com/news/nrx-pharmaceuticals-nasdaq-nrxp-reports-200100848.html
View info-news.info by Front-Page_News