“We were able to show the first full neural control of bionic walking,” said Hyungeun Song, first author of the study and a postdoctoral researcher at MIT.
Most state-of-the art bionic prostheses rely on preprogrammed robotic commands instead of the user’s brain signals. Advanced robotic technologies can sense the environment and repeatedly activate a predefined leg motion to help a person navigate that kind of terrain.
But many of these robotics work best on level ground and struggle to navigate common obstacles such as bumps or puddles. The person wearing the prosthesis often has little say in adjusting the prosthetic limb once it is in motion, especially in response to sudden terrain changes.
“When I walk, it feels like I’m being walked because an algorithm is sending commands to a motor, and I’m not,” said Hugh Herr, principal investigator of the study and professor of media arts and sciences at MIT and a pioneer in the field of biomechatronics, a field that melds biology with electronics and mechanics. Herr’s legs were amputated below the knee several years ago because of frostbite, and he uses advanced robotic prostheses.
“There’s a growing body of evidence [showing] that when you link the brain to a mechatronic prosthesis, there’s an embodiment that occurs where the individual views the synthetic limb as a natural extension of their body,” Herr said.
The authors worked with 14 study participants, half of whom received below-knee amputations through an approach known as the Agonist-antagonist Myoneural Interface — AMI — while the other half underwent traditional amputations.
“What’s super cool about this is how it’s leveraging surgical innovation along with technological innovation,” said Conor Walsh, professor at the Harvard School of Engineering and Applied Sciences who specializes in the development of wearable assistive robots and was not involved in the study.
The AMI amputation was developed to address the limitations of traditional leg amputation surgery, which severs important muscle connections at the amputation site.
Movements are made possible by the way muscles move in pairs. One muscle — known as the agonist — contracts to move a limb and another — known as the antagonist — will lengthen in response. For example, during a biceps curl, the biceps muscle is the agonist because it contracts to lift the forearm up, while the triceps muscle is the antagonist because it lengthens to enable the motion.
When surgical amputation severs muscle pairs, a patient’s ability to feel muscle contractions post-surgery is impaired, and as a result, compromises their ability to accurately and finely sense where their prosthetic limb is in space.
In contrast, the AMI procedure reconnects muscles in the remaining limb to replicate the valuable muscular feedback a person gets from an intact limb.
The study “is part of a movement of the next generation of prosthetic technologies that address sensation and not just movement,” said Eric Rombokas, assistant professor of mechanical engineering at the University of Washington who was not involved in the study.
The AMI procedure for below-knee amputation was named the Ewing Amputation after Jim Ewing, the first person to receive the procedure in 2016.
Patients who underwent the Ewing Amputation experienced less muscle atrophy in their residual limb and less phantom pain, the sensation of experiencing discomfort in a limb that no longer exists.
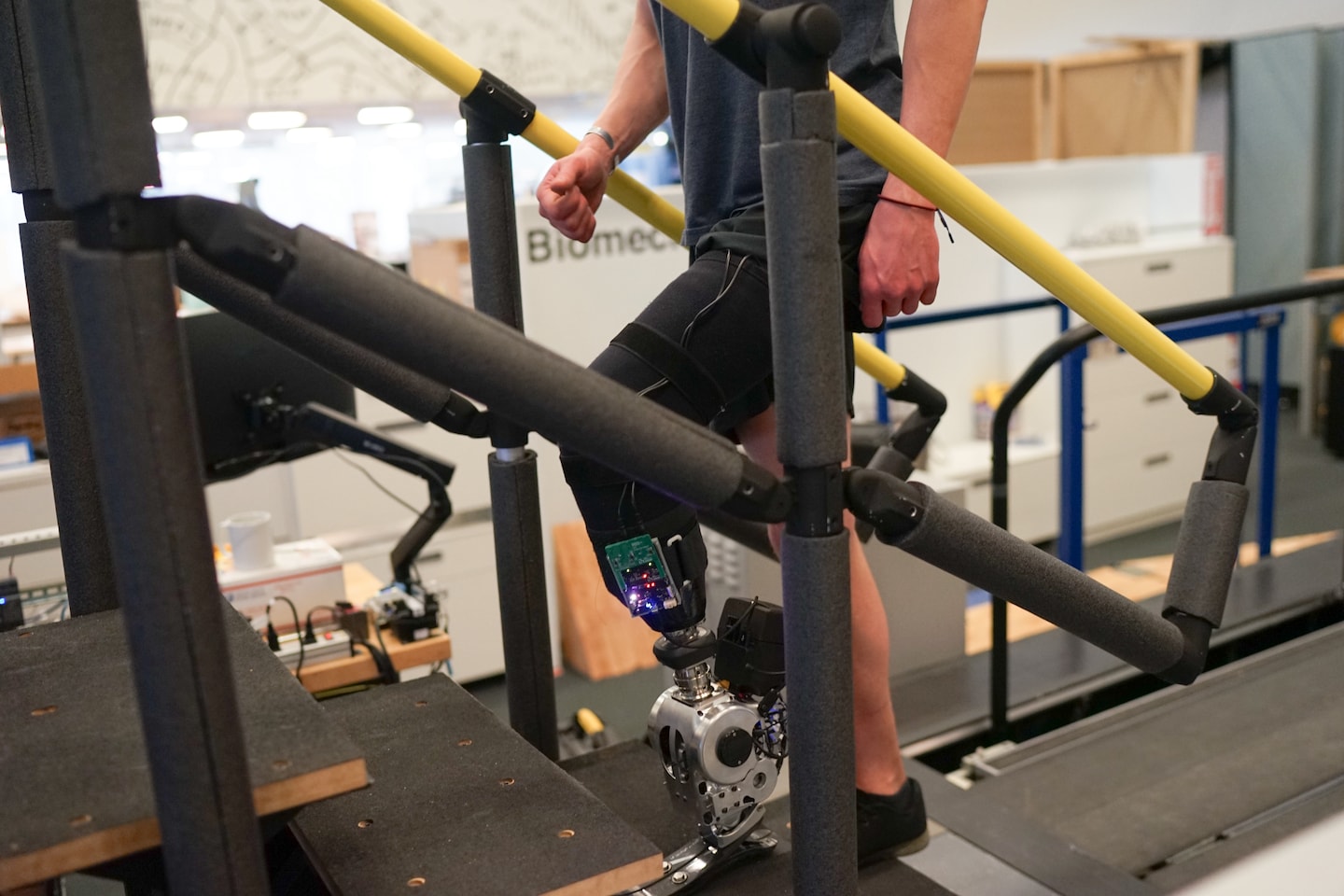
The researchers fit all participants with a novel bionic limb, which consisted of a prosthetic ankle, a device that measures electrical activity from muscle movement and electrodes placed on the surface of the skin.
The brain sends electrical pulses to the muscles, causing them to contract. The contractions produce their own electrical signals, which are detected by the electrodes and sent to small computers on the prosthesis. The computers then convert those electrical signals into force and movement for the prosthesis.
Amy Pietrafitta, a participant in the study who received the Ewing Amputation after severe burn injuries, said the bionic limb gave her the ability to point both of her feet and perform dance moves again.
“Being able to have that type of flexion made it so much more real,” Pietrafitta said. “It felt like everything was there.”
With their enhanced muscle sensations, participants who underwent the Ewing Amputation were able to use their bionic limb to walk faster and with a more natural gait than those who underwent traditional amputations.
When a person has to deviate from normal walking patterns, they typically have to work harder to get around.
“That energy expenditure … causes our heart to work harder and our lungs to work harder … and it can lead to gradual destruction of our hip joints or our lower spine,” said Matthew J. Carty, a reconstructive plastic surgeon at Brigham and Women’s Hospital and the first doctor to perform the AMI procedure.
Patients who received the Ewing Amputation and the new prosthetic limb were also able to easily navigate ramps and stairs. They smoothly adjusted their footing to push themselves up the stairs and absorb shock as they went down.
The researchers hope the novel prosthesis will be commercially available in the next five years.
“We’re starting to get a glimpse of this glorious future wherein a person can lose a major part of their body, and there’s technology available to reconstruct that aspect of their body to full functionality,” Herr said.
#Researchers #develop #braindriven #prosthesis #people #leg #amputations,
#Researchers #develop #braindriven #prosthesis #people #leg #amputations